Allen Laboratory
CONTACT INFO
Irving Coy Allen, MBA, PhD
Department of Biomedical Sciences and Pathobiology
Phone: 540-231-7551
Email: icallen@vt.edu
-
Bio Item
 Irving Coy Allen, PhD, MBA, MS , bio
Irving Coy Allen, PhD, MBA, MS , bioAssistant Head for Research Support Professor, Inflammatory Disease
About the lab
What are the critical factors associated with the initiation and resolution of inflammation? Likewise, dysregulated inflammation is directly associated with a myriad of human and veterinary diseases, including cancer. Indeed dysregulation of inflammation is a hallmark feature of cancer and is a critical element associated with the tumor microenvironment. How can we control or modulate the immune system during tumorigenesis and maintain immune system homeostasis to improve patient outcomes?
These are the questions that my laboratory is attempting to address. As an immunologist, my research program is focused on exploring the intersection between the immune system and cancer. Specifically, we are interested in understanding the contribution of unique families of pattern recognition receptors (PRRs) in modulating disease pathogenesis and inflammatory microenvironments.
Pattern recognition receptors (PRRs)
PRRs are proteins that recognize pathogen-associated molecular patterns (PAMPs), which are present within viruses, bacteria, and other microbial species. PRRs are also responsible for sensing damage-associated molecular patterns (DAMPs), which are produced by host cells under a variety of pathologic conditions to coordinate the immune response to cellular damage and/or stress. PAMPs and DAMPs are recognized by three major classes of PRRs studied in my laboratory:
- The Toll-like receptors (TLRs);
- Retinoic acid inducible gene-I (RIG-I)-like receptors (RLRs); and
- The nucleotide-binding domain-leucine-rich repeat-containing molecules ("NOD-like" receptors; NLRs).
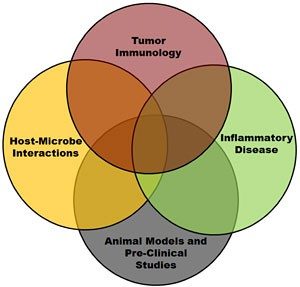
These three protein families and their respective signaling cascades form the foundation of the innate immune system and play critical roles in protecting the host. This line of research has resulted in several high impact publications, including multiple first author or corresponding author manuscripts in Immunity, The Journal of Experimental Medicine, and The Journal of Immunology.
In addition to my research interests in innate immune system signaling and function, I have spent the vast majority of my scientific career developing pre-clinical animal models to study human diseases. Indeed, my laboratory is ideally situated at the interface between basic science research and clinical studies. We have broad experience in novel in vitro/ex vivo modeling, rodent and large mammal pre-clinical models, and studies in human patients.
-
Article Item
-
Article Item
-
Article Item
-
Article Item
-
Article Item
-
Article Item
-
Article Item
-
Article Item
-
Article Item
-
Article Item


